Theme: Developing the Synergy between Pharmaceutics and Medicinal Chemistry to Deliver Better Drugs
Renowned Speakers
Med Pharma Congress 2017
ConferenceSeries Ltd invites all the participants across the globe to attend 16th Annual Pharmaceutical Sciences and Medicinal Chemistry Congress during October 16-17, 2017 in Seoul, South Korea, which includes prompt keynote presentations, Oral talks, Poster presentations and Exhibitions. Med Pharma Congress is a specially designed cluster Pharma conference. The main theme of this Pharma conferences is “Developing the Synergy between Pharmaceutics and Medicinal Chemistry to Deliver Better Drugs” which covers a wide range of critically important sessions.
Track 1: Pharmaceutical sciences:
The pharmaceutical sciences are a group of interdisciplinary areas of study concerned with the design, action, delivery and disposition of drugs. They apply knowledge from chemistry (inorganic, physical, #biochemical and #analytical), biology (anatomy, physiology, biochemistry, cell biology and molecular), #epidemiology, statistics, chemo metrics, mathematics, physics, and chemical engineering.
As new discoveries advance and extend the pharmaceutical sciences, subspecialties continue to be added to this list. Importantly, as knowledge advances, boundaries between these specialty areas of pharmaceutical sciences are beginning to blur. Many fundamental concepts are common to all pharmaceutical sciences. These shared fundamental concepts further the understanding of their applicability to all aspects of pharmaceutical research and drug therapy.
Related Pharma Conferences | Pharma meetings | pharmaceutical conferences
8th Asian Biosimilars Congress, August 14-16, 2017 Osaka, Japan, 11th International Conference on Pharmacoepidemiology and Clinical research, July 06-08, 2017 at Kuala Lumpur, Malaysia, 9th World Congress on BA/BE Studies and Bio waivers scheduled to be held July 17-19, 2017 at Melbourne, Australia, 8th Global Pharmacovigilance & Drug Safety Summit, April 17-18, 2017 Bali, Indonesia, 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea, 8th World Congress on Toxicology and Pharmacology, April 13-15, 2017 Dubai, UAE, 19th International Conference on Advanced Pharmaceutical Engineering and Pharmacology, July 22-23, Porto, Portugal, 18th International Conference on Industrial Pharmacy, December 26-27, 2016, Dubai, UAE, 18th International Conference on Pharmacy and Pharmacology, September 29-30, 2016, 19th International Conference on Pharmacology and Drug Delivery Systems, February 7-8, 2017, Amsterdam, Netherlands, 19th International Conference on Pharmacovigilance and Drug Safety, May 25-26, 2017, London, United Kingdom, 19th International Conference on Ocular Pharmacology and Clinical Pharmacy, January 12-13, 2017, Durban, South Africa, 14th Global Experts Meeting on Nano materials and Nanotechnology, Mar 30-31, 2017, Madrid, Spain
Track 2: Pharmacology and Pharmacovigilance:
Pharmacology is the branch of medicine and biology concerned with the study of #drug action, where a drug can be broadly defined as any man-made, natural, or #endogenous (from within body) molecule which exerts a biochemical and/or physiological effect on the #cell, tissue, organ, or #organism (sometimes the word pharmacon is used as a term to encompass these endogenous and exogenous bioactive species). More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function. If substances have medicinal properties, they are considered pharmaceuticals.
Pharmacovigilance, also known as drug safety, is the #pharmacologicalscience relating to the collection, detection, assessment, monitoring, and prevention of adverse effects with pharmaceutical products. The #etymological roots for the word "pharmacovigilance" are: pharmakon (Greek for drug) and vigilare (Latin for to keep watch). As such, pharmacovigilance heavily focuses on adverse drug reactions, or ADRs, which are defined as any response to a drug which is noxious and unintended, including lack of efficacy (the condition that this definition only applies with the doses normally used for the prophylaxis, diagnosis or therapy of disease, or for the modification of #physiological disorder function was excluded with the latest amendment of the applicable legislation). Medication errors such as overdose, and misuse and abuse of a drug as well as drug exposure during pregnancy and breast feeding, are also of interest, even without an adverse event, because they may result in an adverse drug reaction.
Related Pharma Conferences | Pharma meetings | pharmaceutical conferences
8th Asian Biosimilars Congress, August 14-16, 2017 Osaka, Japan, 11th International Conference on Pharmacoepidemiology and Clinical research, July 06-08, 2017 at Kuala Lumpur, Malaysia, 9th World Congress on BA/BE Studies and Bio waivers scheduled to be held July 17-19, 2017 at Melbourne, Australia, 8th Global Pharmacovigilance & Drug Safety Summit, April 17-18, 2017 Bali, Indonesia, 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea, 8th World Congress on Toxicology and Pharmacology, April 13-15, 2017 Dubai, UAE, 19th International Conference on Advanced Pharmaceutical Engineering and Pharmacology, July 22-23, Porto, Portugal, 18th International Conference on Industrial Pharmacy, December 26-27, 2016, Dubai, UAE, 18th International Conference on Pharmacy and Pharmacology, September 29-30, 2016, 19th International Conference on Pharmacology and Drug Delivery Systems, February 7-8, 2017, Amsterdam, Netherlands, 19th International Conference on Pharmacovigilance and Drug Safety, May 25-26, 2017, London, United Kingdom, 19th International Conference on Ocular Pharmacology and Clinical Pharmacy, January 12-13, 2017, Durban, South Africa, 14th Global Experts Meeting on Nano materials and Nanotechnology, Mar 30-31, 2017, Madrid, Spain
Track 3: Pharmaceutical Engineering
Pharmaceutical engineering is a branch of pharmaceutical science and technology that involves development and manufacturing of products, processes, and components in the pharmaceuticals industry. While developing pharmaceutical products involves many interrelated disciplines like medicinal chemists, analytical chemists, clinicians/pharmacologists, pharmacists, chemical engineers, biomedical engineers, etc, the specific subfield of "pharmaceutical engineering" has only emerged recently as a distinct engineering discipline. This now brings the problem-solving principles and quantitative training of engineering to complement the other scientific fields already involved in drug development.
Pharmaceutical engineering is a branch of pharmaceutical science and technology that involves development and manufacturing of products, processes, and components in the pharmaceuticals industry.
Related Pharmaceutical Engineering conferences | Pharmaceutical Meetings | Biomedical Conferences
8th Asian Biosimilars Congress, August 14-16, 2017 Osaka, Japan, 11th International Conference on Pharmacoepidemiology and Clinical research, July 06-08, 2017 at Kuala Lumpur, Malaysia, 9th World Congress on BA/BE Studies and Bio waivers scheduled to be held July 17-19, 2017 at Melbourne, Australia, 8th Global Pharmacovigilance & Drug Safety Summit, April 17-18, 2017 Bali, Indonesia, 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea, 8th World Congress on Toxicology and Pharmacology, April 13-15, 2017 Dubai, UAE, 19th International Conference on Advanced Pharmaceutical Engineering and Pharmacology, July 22-23, Porto, Portugal, 18th International Conference on Industrial Pharmacy, December 26-27, 2016, Dubai, UAE, 18th International Conference on Pharmacy and Pharmacology, September 29-30, 2016, 19th International Conference on Pharmacology and Drug Delivery Systems, February 7-8, 2017, Amsterdam, Netherlands, 19th International Conference on Pharmacovigilance and Drug Safety, May 25-26, 2017, London, United Kingdom, 19th International Conference on Ocular Pharmacology and Clinical Pharmacy, January 12-13, 2017, Durban, South Africa, 14th Global Experts Meeting on Nano materials and Nanotechnology, Mar 30-31, 2017, Madrid, Spain
Track 4: Bio Pharmaceuticals:
A biopharmaceutical, also known as a biological medical product, biological, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semi synthesized from biological sources. Different from totally synthesized pharmaceuticals, they include vaccines, blood, blood components, allergenic, somatic cells, gene therapies, tissues, recombinant therapeutic protein, and living cells used in cell therapy. Biologics can be composed of sugars, proteins, or nucleic acids or complex combinations of these substances, or may be living cells or tissues. They (or their precursors or components) are isolated from living sources—human, animal, plant, fungal, or microbial.
Terminology surrounding biopharmaceuticals varies between groups and entities, with different terms referring to different subsets of therapeutics within the general biopharmaceutical category. Some regulatory agencies use the terms biological medicinal products or therapeutic biological product to refer specifically to engineered macromolecular products like protein- and nucleic acid-based drugs, distinguishing them from products like blood, blood components, or vaccines, which are usually extracted directly from a biological source. Specialty drugs, a recent classification of pharmaceuticals, are high-cost drugs that are often biologics. Gene-based and cellular biologics, for example, often are at the forefront of biomedical research, and may be used to treat a variety of medical conditions for which no other treatments are available.
Related Pharmacology Conferences | Drug Safety Events | Clinical Pharmacy Meetings
8th Asian Biosimilars Congress, August 14-16, 2017 Osaka, Japan, 11th International Conference on Pharmacoepidemiology and Clinical research, July 06-08, 2017 at Kuala Lumpur, Malaysia, 9th World Congress on BA/BE Studies and Bio waivers scheduled to be held July 17-19, 2017 at Melbourne, Australia, 8th Global Pharmacovigilance & Drug Safety Summit, April 17-18, 2017 Bali, Indonesia, 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea, 8th World Congress on Toxicology and Pharmacology, April 13-15, 2017 Dubai, UAE, 19th International Conference on Advanced Pharmaceutical Engineering and Pharmacology, July 22-23, Porto, Portugal, 18th International Conference on Industrial Pharmacy, December 26-27, 2016, Dubai, UAE, 18th International Conference on Pharmacy and Pharmacology, September 29-30, 2016, 19th International Conference on Pharmacology and Drug Delivery Systems, February 7-8, 2017, Amsterdam, Netherlands, 19th International Conference on Pharmacovigilance and Drug Safety, May 25-26, 2017, London, United Kingdom, 19th International Conference on Ocular Pharmacology and Clinical Pharmacy, January 12-13, 2017, Durban, South Africa, 14th Global Experts Meeting on Nano materials and Nanotechnology, Mar 30-31, 2017, Madrid, Spain
Track 5: Pharmacogenomics
Pharmacogenomics is the study of the role of the #genome in drug response. Its name reflects it’s combining of pharmacology and genomics. Pharmacogenomics can be defined as the technology that analyzes how the genetic makeup of an individual affects his/her response to drugs. It deals with the influence of acquired and inherited genetic variation on drug response in patients by correlating gene expression or single-#nucleotide polymorphisms with #pharmacokinetics and #pharmacodynamics (drug absorption, distribution, metabolism, and elimination), as well as drug receptor target effects. The term pharmacogenomics is often used interchangeably with pharmacogenetics. Although both terms relate to drug response based on genetic influences, pharmacogenetics focuses on single drug-gene interactions, while pharmacogenomics encompasses a more genome-wide association approach, incorporating genomics and epigenetics while dealing with the effects of multiple genes on drug response.
Pharmacogenomics aims to develop rational means to optimize drug therapy, with respect to the patients' genotype, to ensure maximum efficacy with minimal adverse effects. Through the utilization of pharmacogenomics, it is hoped that pharmaceutical drug treatments can deviate from what is dubbed as the "one-dose-fits-all" approach. It attempts to eliminate the trial-and-error method of prescribing, allowing physicians to take into consideration their patient's genes, the functionality of these genes, and how this may affect the efficacy of the patient's current or future treatments (and where applicable, provide an explanation for the failure of past treatments). Such approaches promise the advent of precision medicine and even personalized medicine, in which drugs and drug combinations are optimized for narrow subsets of patients or even for each individual's unique genetic makeup. Whether used to explain a patient's response or lack thereof to a treatment, or act as a predictive tool, it hopes to achieve better treatment outcomes, greater efficacy, minimization of the occurrence of drug toxicities and adverse drug reactions (ADRs). For patients who have lack of therapeutic response to a treatment, alternative therapies can be prescribed that would best suit their requirements. In order to provide pharmacogenomics recommendations for a given drug, two possible types of input can be used: genotyping or exome or whole genome sequencing. Sequencing provides many more data points, including detection of mutations that prematurely terminate the synthesized protein.
Related Pharma Conferences | Pharma meetings | pharmaceutical conferences
8th Asian Biosimilars Congress, August 14-16, 2017 Osaka, Japan, 11th International Conference on Pharmacoepidemiology and Clinical research, July 06-08, 2017 at Kuala Lumpur, Malaysia, 9th World Congress on BA/BE Studies and Bio waivers scheduled to be held July 17-19, 2017 at Melbourne, Australia, 8th Global Pharmacovigilance & Drug Safety Summit, April 17-18, 2017 Bali, Indonesia, 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea, 8th World Congress on Toxicology and Pharmacology, April 13-15, 2017 Dubai, UAE, 19th International Conference on Advanced Pharmaceutical Engineering and Pharmacology, July 22-23, Porto, Portugal, 18th International Conference on Industrial Pharmacy, December 26-27, 2016, Dubai, UAE, 18th International Conference on Pharmacy and Pharmacology, September 29-30, 2016, 19th International Conference on Pharmacology and Drug Delivery Systems, February 7-8, 2017, Amsterdam, Netherlands, 19th International Conference on Pharmacovigilance and Drug Safety, May 25-26, 2017, London, United Kingdom, 19th International Conference on Ocular Pharmacology and Clinical Pharmacy, January 12-13, 2017, Durban, South Africa, 14th Global Experts Meeting on Nano materials and Nanotechnology, Mar 30-31, 2017, Madrid, Spain
Track 6: Pharmacognosy and Phytochemistry
#Pharmacognosy is the study of medicinal drugs derived from plants or other natural sources. The American Society of Pharmacognosy defines Pharmacognosy as "the study of the physical, chemical, #biochemical and biological properties of drugs, drug substances or potential drugs or drug substances of natural origin as well as the search for new drugs from natural sources. It is also defined as the study of crude drugs.
#Phytochemistry is the study of phytochemicals, which are chemicals derived from plants. Specifically, Phytochemistry describes the large number of secondary metabolic compounds found in plants. Many of these are known to provide protection against insect attacks and plant #diseases. They also exhibit a number of protective functions for human consumers. Phytochemistry can be considered sub-fields of botany or chemistry. Activities can be led in botanical gardens or in the wild with the aid of ethno botany. The applications of the discipline can be for Pharmacognosy, or the discovery of new drugs, or as an aid for plant physiology studies
Global sales of plant products was totally estimated us $60 billion in 2002 and is expected to get higher at 6.4 % average growth rate
Related Pharma Conferences | Pharma meetings | pharmaceutical conferences
8th Asian Biosimilars Congress, August 14-16, 2017 Osaka, Japan, 11th International Conference on Pharmacoepidemiology and Clinical research, July 06-08, 2017 at Kuala Lumpur, Malaysia, 9th World Congress on BA/BE Studies and Bio waivers scheduled to be held July 17-19, 2017 at Melbourne, Australia, 8th Global Pharmacovigilance & Drug Safety Summit, April 17-18, 2017 Bali, Indonesia, 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea, 8th World Congress on Toxicology and Pharmacology, April 13-15, 2017 Dubai, UAE, 19th International Conference on Advanced Pharmaceutical Engineering and Pharmacology, July 22-23, Porto, Portugal, 18th International Conference on Industrial Pharmacy, December 26-27, 2016, Dubai, UAE, 18th International Conference on Pharmacy and Pharmacology, September 29-30, 2016, 19th International Conference on Pharmacology and Drug Delivery Systems, February 7-8, 2017, Amsterdam, Netherlands, 19th International Conference on Pharmacovigilance and Drug Safety, May 25-26, 2017, London, United Kingdom, 19th International Conference on Ocular Pharmacology and Clinical Pharmacy, January 12-13, 2017, Durban, South Africa, 14th Global Experts Meeting on Nano materials and Nanotechnology, Mar 30-31, 2017, Madrid, Spain
Track 7: Pharmaceutical Toxicology
Pharmaceutical #Toxicology explains the #methodology and requirements of #pre-clinical safety assessments of new medicines. With the focus on medicinal drugs, the most important safety issues of drugs are covered. This includes registration requirements of new drugs and #pharmacovigilance.
Pharmaceutical Toxicology is focusing on the diagnosis, management and prevention of poisoning and other adverse health effects due to medications, occupational and environmental toxicants, and biological agents. Medical toxicologists are involved in the assessment and treatment of acute or #chronic poisoning, adverse drug reactions (ADR), overdoses, envenomation’s, and substance abuse, and other chemical exposures.
Related Toxicology Meetings | Toxicology congress | Toxicology congress
8th Asian Biosimilars Congress, August 14-16, 2017 Osaka, Japan, 11th International Conference on Pharmacoepidemiology and Clinical research, July 06-08, 2017 at Kuala Lumpur, Malaysia, 9th World Congress on BA/BE Studies and Bio waivers scheduled to be held July 17-19, 2017 at Melbourne, Australia, 8th Global Pharmacovigilance & Drug Safety Summit, April 17-18, 2017 Bali, Indonesia, 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea, 8th World Congress on Toxicology and Pharmacology, April 13-15, 2017 Dubai, UAE, 19th International Conference on Advanced Pharmaceutical Engineering and Pharmacology, July 22-23, Porto, Portugal, 18th International Conference on Industrial Pharmacy, December 26-27, 2016, Dubai, UAE, 18th International Conference on Pharmacy and Pharmacology, September 29-30, 2016, 19th International Conference on Pharmacology and Drug Delivery Systems, February 7-8, 2017, Amsterdam, Netherlands, 19th International Conference on Pharmacovigilance and Drug Safety, May 25-26, 2017, London, United Kingdom, 19th International Conference on Ocular Pharmacology and Clinical Pharmacy, January 12-13, 2017, Durban, South Africa, 14th Global Experts Meeting on Nano materials and Nanotechnology, Mar 30-31, 2017, Madrid, Spain
Track 8: Industrial Pharmacy
Industrial #Pharmacy also plays a crucial role in any drug discovery. To any novel drug discovery the #industrial approach is very important to get massive commercial application. Few things which have to be considered by industries to provide a safe and cost affective medicine to the patients like Supply chain, Waste management, Product management, #Post- marketing surveillance, Good manufacturing practices and #Marketing.
The U.S. pharmaceutical market is the world’s most important national market. Together with Canada and Mexico, it represents the largest continental Pharma market worldwide. The United States alone holds some 40 percent of the global pharmaceutical market. In 2014, this share was valued around 365 million U.S. dollars. Many of the global top companies are located in the United States. In 2014, six out of the top eleven companies were U.S.-based.
Related Pharmaceutical Engineering conferences| Pharmaceutical Meetings| Biomedical Conferences
8th Asian Biosimilars Congress, August 14-16, 2017 Osaka, Japan, 11th International Conference on Pharmacoepidemiology and Clinical research, July 06-08, 2017 at Kuala Lumpur, Malaysia, 9th World Congress on BA/BE Studies and Bio waivers scheduled to be held July 17-19, 2017 at Melbourne, Australia, 8th Global Pharmacovigilance & Drug Safety Summit, April 17-18, 2017 Bali, Indonesia, 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea, 8th World Congress on Toxicology and Pharmacology, April 13-15, 2017 Dubai, UAE, 19th International Conference on Advanced Pharmaceutical Engineering and Pharmacology, July 22-23, Porto, Portugal, 18th International Conference on Industrial Pharmacy, December 26-27, 2016, Dubai, UAE, 18th International Conference on Pharmacy and Pharmacology, September 29-30, 2016, 19th International Conference on Pharmacology and Drug Delivery Systems, February 7-8, 2017, Amsterdam, Netherlands, 19th International Conference on Pharmacovigilance and Drug Safety, May 25-26, 2017, London, United Kingdom, 19th International Conference on Ocular Pharmacology and Clinical Pharmacy, January 12-13, 2017, Durban, South Africa, 14th Global Experts Meeting on Nano materials and Nanotechnology, Mar 30-31, 2017, Madrid, Spain
Track 9: Regulatory Affairs
#Regulatory affairs (RA), is also called government affairs, is a profession within regulated industries, such as pharmaceuticals, medical devices, energy, banking, telecom etc. Regulatory affairs also has a very specific meaning within the #healthcare industries (pharmaceuticals, #medical devices, #biologics and functional foods)
Regulatory affairs (medical affairs) professionals (aka regulatory professionals) usually have responsibility for the following general areas: Ensuring that their companies comply with all of the regulations and laws pertaining to their business, Working with federal, state and local regulatory agencies and personnel on specific issues affecting their business. i.e. working with such agencies as the Food and Drug Administration or European Medicines Agency (pharmaceuticals and medical devices); The Department of Energy; or the Securities and Exchange Commission (banking), Advising their companies on the regulatory aspects and climate that would affect proposed activities. i.e. describing the "regulatory climate" around issues such as the promotion of prescription drugs and Sarbanes-Oxley compliance.
Related Pharmacology Conferences | Drug Safety Events | Clinical Pharmacy Meetings
8th Asian Biosimilars Congress, August 14-16, 2017 Osaka, Japan, 11th International Conference on Pharmacoepidemiology and Clinical research, July 06-08, 2017 at Kuala Lumpur, Malaysia, 9th World Congress on BA/BE Studies and Bio waivers scheduled to be held July 17-19, 2017 at Melbourne, Australia, 8th Global Pharmacovigilance & Drug Safety Summit, April 17-18, 2017 Bali, Indonesia, 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea, 8th World Congress on Toxicology and Pharmacology, April 13-15, 2017 Dubai, UAE, 19th International Conference on Advanced Pharmaceutical Engineering and Pharmacology, July 22-23, Porto, Portugal, 18th International Conference on Industrial Pharmacy, December 26-27, 2016, Dubai, UAE, 18th International Conference on Pharmacy and Pharmacology, September 29-30, 2016, 19th International Conference on Pharmacology and Drug Delivery Systems, February 7-8, 2017, Amsterdam, Netherlands, 19th International Conference on Pharmacovigilance and Drug Safety, May 25-26, 2017, London, United Kingdom, 19th International Conference on Ocular Pharmacology and Clinical Pharmacy, January 12-13, 2017, Durban, South Africa, 14th Global Experts Meeting on Nano materials and Nanotechnology, Mar 30-31, 2017, Madrid, Spain
Track 10: Pharmaceutical Formulation
Pharmaceutical #formulation, in pharmaceutics, is the process in which different chemical substances, including the active drug, are combined to produce a final medicinal product. The word formulation is often used in a way that includes #dosage form. Formulation studies involve developing a #preparation of the drug which is both stable and acceptable to the #patient. For orally administered drugs, this usually involves incorporating the drug into a tablet or a capsule. It is important to make the distinction that a tablet contains a variety of other potentially inert substances apart from the drug itself, and studies have to be carried out to ensure that the encapsulated drug is compatible with these other substances in a way that does not cause harm, whether direct or indirect.
Preformulation involves the characterization of a drug's physical, chemical, and mechanical properties in order to choose what other ingredients (excipients) should be used in the preparation. In dealing with protein pre-formulation, the important aspect is to understand the solution behavior of a given protein under a variety of stress conditions such as freeze/thaw, temperature, shear stress among others to identify mechanisms of degradation and therefore its mitigation. Formulation studies then consider such factors as particle size, polymorphism, PH, and solubility, as all of these can influence bioavailability and hence the activity of a drug. The drug must be combined with inactive ingredients by a method which ensures that the quantity of drug present is consistent in each dosage unit e.g. each tablet. The dosage should have a uniform appearance, with an acceptable taste, tablet hardness, or capsule disintegration.
Related Medicinal Chemistry Conferences | Medicine Conferences | medchem conferences
8th Asian Biosimilars Congress, August 14-16, 2017 Osaka, Japan, 11th International Conference on Pharmacoepidemiology and Clinical research, July 06-08, 2017 at Kuala Lumpur, Malaysia, 9th World Congress on BA/BE Studies and Bio waivers scheduled to be held July 17-19, 2017 at Melbourne, Australia, 8th Global Pharmacovigilance & Drug Safety Summit, April 17-18, 2017 Bali, Indonesia, 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea, 8th World Congress on Toxicology and Pharmacology, April 13-15, 2017 Dubai, UAE, 19th International Conference on Advanced Pharmaceutical Engineering and Pharmacology, July 22-23, Porto, Portugal, 18th International Conference on Industrial Pharmacy, December 26-27, 2016, Dubai, UAE, 18th International Conference on Pharmacy and Pharmacology, September 29-30, 2016, 19th International Conference on Pharmacology and Drug Delivery Systems, February 7-8, 2017, Amsterdam, Netherlands, 19th International Conference on Pharmacovigilance and Drug Safety, May 25-26, 2017, London, United Kingdom, 19th International Conference on Ocular Pharmacology and Clinical Pharmacy, January 12-13, 2017, Durban, South Africa, 14th Global Experts Meeting on Nano materials and Nanotechnology, Mar 30-31, 2017, Madrid, Spain
Track 11: Nano Medicine
Nano medicine is the medical application of nanotechnology. Nano medicine ranges from the medical applications of #nanomaterial’s and biological devices, to Nano electronic biosensors, and even possible future applications of molecular #nanotechnology such as biological machines. Current problems for Nano medicine involve understanding the issues related to toxicity and environmental impact of Nano scale materials (materials whose structure is on the scale of nanometers, i.e. billionths of a meter). Functionalities can be added to nanomaterial by interfacing them with biological molecules or structures. The size of nanomaterial’ is similar to that of most biological molecules and structures; therefore, #nanomaterial’s can be useful for both in vivo and in vitro biomedical research and applications. Thus far, the integration of nanomaterial with biology has led to the development of #diagnostic devices, contrast agents, analytical tools, physical therapy applications, and drug delivery vehicles.
Nano medicine seeks to deliver a valuable set of research tools and clinically useful devices in the near future. The National Nanotechnology Initiative expects new commercial applications in the pharmaceutical industry that may include advanced drug delivery systems, new therapies, and in vivo imaging. Nano medicine research is receiving funding from the US National Institutes of Health, including the funding in 2005 of a five-year plan to set up four Nano medicine centers. Nano medicine sales reached $16 billion in 2015, with a minimum of $3.8 billion in nanotechnology R&D being invested every year. Global funding for emerging nanotechnology is increased by 45% per year in recent years, with product sales exceeding $1 trillion in 2013. As the Nano medicine industry continues to grow, it is expected to have a significant impact on the economy.
Related Pharmaceutical Engineering conferences| Pharmaceutical Meetings| Biomedical Conferences
8th Asian Biosimilars Congress, August 14-16, 2017 Osaka, Japan, 11th International Conference on Pharmacoepidemiology and Clinical research, July 06-08, 2017 at Kuala Lumpur, Malaysia, 9th World Congress on BA/BE Studies and Bio waivers scheduled to be held July 17-19, 2017 at Melbourne, Australia, 8th Global Pharmacovigilance & Drug Safety Summit, April 17-18, 2017 Bali, Indonesia, 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea, 8th World Congress on Toxicology and Pharmacology, April 13-15, 2017 Dubai, UAE, 19th International Conference on Advanced Pharmaceutical Engineering and Pharmacology, July 22-23, Porto, Portugal, 18th International Conference on Industrial Pharmacy, December 26-27, 2016, Dubai, UAE, 18th International Conference on Pharmacy and Pharmacology, September 29-30, 2016, 19th International Conference on Pharmacology and Drug Delivery Systems, February 7-8, 2017, Amsterdam, Netherlands, 19th International Conference on Pharmacovigilance and Drug Safety, May 25-26, 2017, London, United Kingdom, 19th International Conference on Ocular Pharmacology and Clinical Pharmacy, January 12-13, 2017, Durban, South Africa, 14th Global Experts Meeting on Nano materials and Nanotechnology, Mar 30-31, 2017, Madrid, Spain
Track 12: Analytical and Bio analytical Techniques
An #analytical technique is a method that is used to determine the #concentration of a chemical compound or chemical element. There are a wide variety of techniques used for analysis, from simple weighing (gravimetric analysis) to titrations (titrimetric) to very advanced techniques using highly specialized instrumentation. Bio analysis is a sub-discipline of analytical chemistry covering the quantitative measurement of xenobiotic (drugs and their #metabolites, and biological molecules in unnatural locations or concentrations) and biotic (#macromolecules, proteins, DNA, large molecule drugs, metabolites) in biological systems.
Many scientific endeavors are dependent upon accurate quantification of drugs and endogenous substances in biological samples; the focus of bio analysis in the pharmaceutical industry is to provide a quantitative measure of the active drug and/or its metabolite(s) for the purpose of pharmacokinetics, toxicokinetics, bioequivalence and exposure–response (pharmacokinetics/pharmacodynamics studies). Bio analysis also applies to drugs used for illicit purposes, forensic investigations, anti-doping testing in sports, and environmental concerns. Bio analysis was traditionally thought of in terms of measuring small molecule drugs. However, the past twenty years has seen an increase in biopharmaceuticals (e.g. proteins and peptides), which have been developed to address many of the same diseases as small molecules. These larger biomolecules have presented their own unique challenges to quantification.
Related Analytical Conferences | Medicinal Chemistry Conferences |Clinical Pharmacy Conferences
8th Asian Biosimilars Congress, August 14-16, 2017 Osaka, Japan, 11th International Conference on Pharmacoepidemiology and Clinical research, July 06-08, 2017 at Kuala Lumpur, Malaysia, 9th World Congress on BA/BE Studies and Bio waivers scheduled to be held July 17-19, 2017 at Melbourne, Australia, 8th Global Pharmacovigilance & Drug Safety Summit, April 17-18, 2017 Bali, Indonesia, 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea, 8th World Congress on Toxicology and Pharmacology, April 13-15, 2017 Dubai, UAE, 19th International Conference on Advanced Pharmaceutical Engineering and Pharmacology, July 22-23, Porto, Portugal, 18th International Conference on Industrial Pharmacy, December 26-27, 2016, Dubai, UAE, 18th International Conference on Pharmacy and Pharmacology, September 29-30, 2016, 19th International Conference on Pharmacology and Drug Delivery Systems, February 7-8, 2017, Amsterdam, Netherlands, 19th International Conference on Pharmacovigilance and Drug Safety, May 25-26, 2017, London, United Kingdom, 19th International Conference on Ocular Pharmacology and Clinical Pharmacy, January 12-13, 2017, Durban, South Africa, 14th Global Experts Meeting on Nano materials and Nanotechnology, Mar 30-31, 2017, Madrid, Spain
Track 13: Drug designing and Drug Delivery
Drug design, often referred to as rational drug design or simply rational design, is the inventive process of finding new #medications based on the knowledge of a biological target. The drug is most commonly an organic small molecule that activates or inhibits the function of a #biomolecule such as a protein, which in turn results in a therapeutic benefit to the patient. In the most basic sense, drug design involves the design of molecules that are complementary in shape and charge to the bio #moleculartarget with which they interact and therefore will bind to it. Drug design frequently but not necessarily relies on computer modeling techniques. This type of modeling is sometimes referred to as computer-aided drug design. Finally, drug design that relies on the knowledge of the three-dimensional structure of the bio molecular target is known as structure-based drug design. In addition to small molecules, biopharmaceuticals and especially therapeutic antibodies are an increasingly important class of drugs and computational methods for improving the affinity, selectivity, and stability of this protein-based therapeutics have also been developed.
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes pre-clinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug.
Related Pharmacology Conferences | Drug Safety Events | Clinical Pharmacy Meetings
8th Asian Biosimilars Congress, August 14-16, 2017 Osaka, Japan, 11th International Conference on Pharmacoepidemiology and Clinical research, July 06-08, 2017 at Kuala Lumpur, Malaysia, 9th World Congress on BA/BE Studies and Bio waivers scheduled to be held July 17-19, 2017 at Melbourne, Australia, 8th Global Pharmacovigilance & Drug Safety Summit, April 17-18, 2017 Bali, Indonesia, 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea, 8th World Congress on Toxicology and Pharmacology, April 13-15, 2017 Dubai, UAE, 19th International Conference on Advanced Pharmaceutical Engineering and Pharmacology, July 22-23, Porto, Portugal, 18th International Conference on Industrial Pharmacy, December 26-27, 2016, Dubai, UAE, 18th International Conference on Pharmacy and Pharmacology, September 29-30, 2016, 19th International Conference on Pharmacology and Drug Delivery Systems, February 7-8, 2017, Amsterdam, Netherlands, 19th International Conference on Pharmacovigilance and Drug Safety, May 25-26, 2017, London, United Kingdom, 19th International Conference on Ocular Pharmacology and Clinical Pharmacy, January 12-13, 2017, Durban, South Africa, 14th Global Experts Meeting on Nano materials and Nanotechnology, Mar 30-31, 2017, Madrid, Spain
Track 14: Clinical Trials and Clinical Pharmacy:
Clinical pharmacy is the branch of pharmacy in which pharmacists provide patient care that optimizes the use of medication and promotes health, wellness, and disease prevention. Clinical pharmacists care for patients in all health care settings but the clinical pharmacy movement initially began inside hospitals and clinics. Clinical pharmacists often work in collaboration with physicians, nurse practitioners, and other healthcare professionals.
Clinical trials are experiments done in clinical research. Such prospective biomedical or behavioral research studies on human participants are designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial - their approval does not mean that the therapy is 'safe' or effective, only that the trial may be conducted.
Related Analytical Conferences | Medicinal Chemistry Conferences |Clinical Pharmacy Conferences
8th Asian Biosimilars Congress, August 14-16, 2017 Osaka, Japan, 11th International Conference on Pharmacoepidemiology and Clinical research, July 06-08, 2017 at Kuala Lumpur, Malaysia, 9th World Congress on BA/BE Studies and Bio waivers scheduled to be held July 17-19, 2017 at Melbourne, Australia, 8th Global Pharmacovigilance & Drug Safety Summit, April 17-18, 2017 Bali, Indonesia, 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea, 8th World Congress on Toxicology and Pharmacology, April 13-15, 2017 Dubai, UAE, 19th International Conference on Advanced Pharmaceutical Engineering and Pharmacology, July 22-23, Porto, Portugal, 18th International Conference on Industrial Pharmacy, December 26-27, 2016, Dubai, UAE, 18th International Conference on Pharmacy and Pharmacology, September 29-30, 2016, 19th International Conference on Pharmacology and Drug Delivery Systems, February 7-8, 2017, Amsterdam, Netherlands, 19th International Conference on Pharmacovigilance and Drug Safety, May 25-26, 2017, London, United Kingdom, 19th International Conference on Ocular Pharmacology and Clinical Pharmacy, January 12-13, 2017, Durban, South Africa, 14th Global Experts Meeting on Nano materials and Nanotechnology, Mar 30-31, 2017, Madrid, Spain
Track 15: Digitization of Pharma:
The pharmaceuticals and life sciences industry has been very cautious in applying digital technology to improve manufacturing and supply chain operations thus far, yet that caution is becoming a hindrance. As the Pharma industry faces growing challenges including globalization, great supply chain complexity, price and cost pressure, and personalized medicine, among others digitization holds tremendous potential in helping companies adapt.
The pharmaceuticals and life sciences industry is generally quite advanced in its application of technology. Regarding operations, however, the industry has been very cautious in adopting new technologies thus far, still relying on supply chain and manufacturing paradigms that have been around for a long time. One key reason is the regulatory environment in which Pharma companies operate. High margins have traditionally been another reason, leading to a stronger focus on new product development and sales rather than on optimizing operations.
Related Pharma Conferences | Pharma meetings | pharmaceutical conferences
8th Asian Biosimilars Congress, August 14-16, 2017 Osaka, Japan, 11th International Conference on Pharmacoepidemiology and Clinical research, July 06-08, 2017 at Kuala Lumpur, Malaysia, 9th World Congress on BA/BE Studies and Bio waivers scheduled to be held July 17-19, 2017 at Melbourne, Australia, 8th Global Pharmacovigilance & Drug Safety Summit, April 17-18, 2017 Bali, Indonesia, 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea, 8th World Congress on Toxicology and Pharmacology, April 13-15, 2017 Dubai, UAE, 19th International Conference on Advanced Pharmaceutical Engineering and Pharmacology, July 22-23, Porto, Portugal, 18th International Conference on Industrial Pharmacy, December 26-27, 2016, Dubai, UAE, 18th International Conference on Pharmacy and Pharmacology, September 29-30, 2016, 19th International Conference on Pharmacology and Drug Delivery Systems, February 7-8, 2017, Amsterdam, Netherlands, 19th International Conference on Pharmacovigilance and Drug Safety, May 25-26, 2017, London, United Kingdom, 19th International Conference on Ocular Pharmacology and Clinical Pharmacy, January 12-13, 2017, Durban, South Africa, 14th Global Experts Meeting on Nano materials and Nanotechnology, Mar 30-31, 2017, Madrid, Spain
Track 16: Intellectual Property Rights (IPR)
Intellectual property (IP) refers to creations of the intellect for which a monopoly is assigned to designated owners by law. Intellectual property rights (#IPR) are the protections granted to the creators of IP, and include trademarks, copyright, #patents, industrial design rights, and in some jurisdictions trade secrets. Artistic works including music and literature, as well as discoveries, inventions, words, phrases, symbols, and designs can all be protected as intellectual property.
While intellectual property law has evolved over centuries, it was not until the 19th century that the term intellectual property began to be used, and not until the late 20th century that it became commonplace in the majority of the world.
Related Pharma Conferences | Pharma meetings | pharmaceutical conferences
8th Asian Biosimilars Congress, August 14-16, 2017 Osaka, Japan, 11th International Conference on Pharmacoepidemiology and Clinical research, July 06-08, 2017 at Kuala Lumpur, Malaysia, 9th World Congress on BA/BE Studies and Bio waivers scheduled to be held July 17-19, 2017 at Melbourne, Australia, 8th Global Pharmacovigilance & Drug Safety Summit, April 17-18, 2017 Bali, Indonesia, 9th Annual Congress on Drug Formulation & Drug Design, October 19-21, 2017 Seoul, South Korea, 8th World Congress on Toxicology and Pharmacology, April 13-15, 2017 Dubai, UAE, 19th International Conference on Advanced Pharmaceutical Engineering and Pharmacology, July 22-23, Porto, Portugal, 18th International Conference on Industrial Pharmacy, December 26-27, 2016, Dubai, UAE, 18th International Conference on Pharmacy and Pharmacology, September 29-30, 2016, 19th International Conference on Pharmacology and Drug Delivery Systems, February 7-8, 2017, Amsterdam, Netherlands, 19th International Conference on Pharmacovigilance and Drug Safety, May 25-26, 2017, London, United Kingdom, 19th International Conference on Ocular Pharmacology and Clinical Pharmacy, January 12-13, 2017, Durban, South Africa, 14th Global Experts Meeting on Nano materials and Nanotechnology, Mar 30-31, 2017, Madrid, Spain
ConferenceSeries Ltd invites all the participants across the globe to attend 16th Annual Medicinal and Pharmaceutical Sciences Congress during October 16-17, 2017 in Seoul, South Korea which includes prompt keynote presentations, Oral talks, Poster presentations and Exhibitions. Med Pharma Congress is a specially designed cluster Pharmaceutical and Medicinal Chemistry Conference. The main theme of this Pharma conferences is “ Developing the Synergy between Pharmaceutics and Medicinal Chemistry to Deliver Better Drugs” which covers a wide range of critically important sessions.
Why to Attend???
Meet Your Target Market with members from around the world focused on learning about Pharmaceutical Sciences and Medicinal Chemistry, this is your single best opportunity to reach the largest assemblage of participants from the Pharma and medicinal community. Conduct demonstrations, distribute information, meet with current and potential Scientists, make a splash with a new research, and receive name recognition at this 2-day Pharmaceutical Science & Medicinal Chemistry. World-renowned speakers, the most recent techniques, tactics, and the newest updates in Pharmaceutical Sciences fields are hallmarks of this conference.
A Unique Opportunity for Advertisers and Sponsors at this International event:
Target Audience:
- Professors, Associate Professors, Assistant Professors
- PhD Scholars
- Graduates and Post Graduates
- Directors, CEO’s of Organizations
- Association, Association presidents and professionals
- Noble laureates in Health Care and Medicine
- Bio instruments Professionals
- Bio-informatics Professionals
- Software development companies
- Research Institutes and members
- Supply Chain companies
- Manufacturing Companies
- CRO and DATA management Companies
- Training Institutes
- Business Entrepreneur
Asia: Pharma’s New Centre of Gravity:-
Asia is poised to become the biggest pharmaceutical market in the world. The increasing amount of R&D and manufacturing that are migrating to Asia are fueling the region’s dynamic growth both as a base to serve the global healthcare market, and as a market in its own right.
Frost & Sullivan forecasts that the global branded Pharma and biotech industry will generate nearly $1.1 trillion in revenue by 2015. Amid Asia’s strong economic growth, coupled with greater access and demand for healthcare, the prospects for Pharma manufacturers in the region look overwhelmingly positive. According to the IMS Consulting Group, the region’s Pharma market is expected to reach $350 billion in 2016, comprising 30% of the global Pharma market and driving close to 50% of global, incremental growth through 2016.
Without a doubt, Asia will lead the growth of the global Pharmaceutical Industry. Alongside this trend, Pharma Asia – the authoritative source of information for pharmaceutical manufacturers – will continue to provide the most compelling and in-depth industry information about the latest trends and technologies of all aspects in drug manufacturing to empower readers to make informed business decisions in tapping market opportunities that Asia has to offer.
Southeast Asia is a growing pharmaceutical market. Much like Latin America, the countries of southeast Asia, especially the members of the Association of Southeast Asian Nations (ASEAN: Brunei, Burma, Cambodia, Indonesia, Laos, Malaysia, the Philippines, Singapore, Thailand, and Vietnam), have taken initial steps towards seeking more harmonized regulation of pharmaceutical and medical-device industries. Despite all the market potential, Asia remains a fiercely competitive region for pharmaceuticals. Part of the source of competition comes from the highly fragmented industry with literally thousands of smaller manufacturers. Many of the larger firms have been able to grow their market share partly through intensive competitive pricing. There are many opportunities for pharmaceutical companies looking to expand in Southeast Asia. As regulation across the region becomes more harmonized and complexity decreases against a backdrop of largely sustained economic growth, the ASEAN region looks increasingly attractive. Understanding the unique demographic and payer mix of each country will be crucial to move forward and take advantage of the opportunities presented in this region
The global market for contract pharmaceutical manufacturing, research and packaging totaled $248.5 billion in 2014 and is projected to approach $352.8 billion by 2019, registering a compound annual growth rate (CAGR) of 7.3% through 2019.
This report provides:
- An overview of the global market for contract pharmaceutical manufacturing, research, and packaging, with emphasis on the current and future outlook of the market and a comprehensive analysis of the overall pharmaceutical and biotechnology outsourcing industry.
- Analyses of global market trends, with data from 2013 and 2014, and projections of compound annual growth rates (CAGRs) through 2019.
- Examination of the effects on market growth of state-of-the-art packaging facilities as well as expensive research facilities and the capabilities of research organizations.
- Coverage of the market's need for outsourcing, the regulatory environment, and technological issues, including the latest trends and developments.
- Comprehensive company profiles of major players.
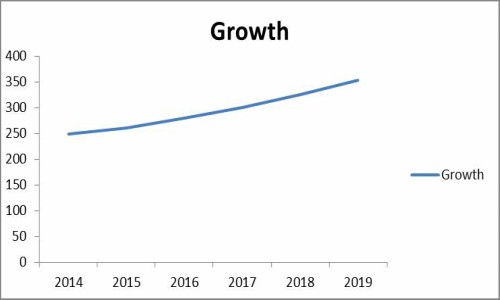
The global market for drug repurposing will grow from nearly $24.4 billion in 2015 to nearly $31.3 billion by 2020, with a compound annual growth rate (CAGR) of 5.1% for the period of 2015-2020.
This report provides:
- An overview of the global markets for drug re-purposing, (also known as re-positioning or re-profiling) referring to studies about a compound or a biologic agent that treats one condition and sees if that particular compound is safe and effective for treating other diseases.
- Analyses of U.S. as well as international market trends, with data from 2014, 2015, and projections of compound annual growth rates (CAGRs) through 2020.
- A look at how human intervention and investigation early in the drug discovery process is helping in faster development of therapeutics.
- Evaluation of drug-repurposing compared to traditional drug development where cost and the time for the drug to be available in the market is greatly reduced since the re-purposed drug has already passed a significant number of tests.
- Coverage of how the discovery process for repurposing drugs has accelerated in recent years due to the emergence of new tools and technologies.
Profiles of major players in the industry
GLOBAL MARKET TRENDS FOR REPURPOSED DRUGS BY REGION, 2013-2020 ($ MILLIONS)
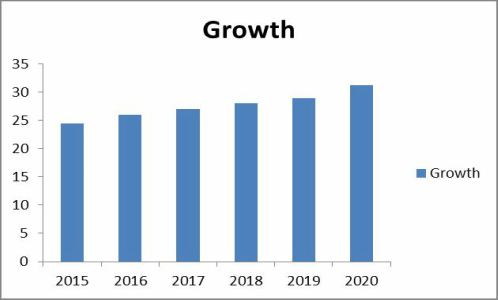
The pharmaceutical market in South Korea will register a slow but steady rise from $18.6 billion in 2016 to $20.4 billion in 2020.
This is according to new market research from analysts at Global Data, who see the compound annual growth rate (CAGR) of meds in the country reaching just 2.4%, as the Korean government increasingly focuses on generics in order to reduce healthcare spend.
The company’s latest report found that the country’s generics market increased from $3.5 billion in 2008 to around $5.8 billion in 2015--growing at a CAGR of 7%--as the government has invested significantly in the generics market in recent years.
The analysts said that other government initiatives will also aid businesses, such as lifting the ban on advertisements for medical services, which has enabled hospitals to hire advertising agencies to help them attract medical tourists
Korea Pharmaceutical Associations:
Korea Pharmaceutical Manufacturers Association KPMA
Korea Pharmaceutical Traders Association KPTA
Korea Pharmaceutical & Bio Science Society Korea PBSS
The Pharmaceutical Society of Korea (PSK)
Pharmaceutical Society of Korea
Asian Pharmaceutical Associations:-
Federation of Asian Pharmaceutical Associations
Asian Association of Schools of Pharmacy
Asia Partnership Conference of Pharmaceutical Associations
Asian Pharmacy Student Association
Commonwealth Pharmacists Association
International Society of Pharmacovigilance
International Pharmaceutical Associations:-
International Federation of Pharmaceutical Manufacturers & Associations (IFPMA)
European Federation of Pharmaceutical Industries and Associations (EFPIA)
Central American Federation of Pharmaceutical Laboratories (FEDEFARMA)
Medicines Australia
Bangladesh Association of Pharmaceutical Industries (BAPI)
Association of International Pharmaceutical Manufacturers (AIPM)
Belgium Pharmaceutical Industry Association
Brazilian Research-Based Pharmaceutical Manufacturers Association (INTERFARMA)
Canada’s Research-Based Pharmaceutical Companies –Rx & D
R&D-Based Pharmaceutical Association in China (RDPAC)
The Hong Kong Association of the Pharmaceutical Industry (HKAPI)
Organization of Pharmaceutical Producers of India (OPPI)
International Pharmaceutical Manufacturers Group (IPMG)
The Association of Research-Based Pharmaceutical Companies (AIFD)
Conference Highlights
- Pharmaceutical sciences
- Pharmacology and Pharmacovigilance
- Pharmaceutical Engineering
- Bio Pharmaceuticals
- Pharmacogenomics
- Pharmacognosy and Phytochemistry
- Pharmaceutical Toxicology
- Industrial Pharmacy
- Regulatory Affairs
- Pharmaceutical Formulation
- Nano Medicine
- Analytical and Bio Analytical Techniques
- Drug designing and Drug Delivery
- Clinical Pharmacy and Clinical Trials
- Intellectual Property Rights (IPR)
- Digitization of Pharma
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | October 16-17, 2017 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | ||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Pharmaceutical Sciences & Emerging Drugs
- Journal of Medicinal Chemistry
- Journal of Drug Designing
Abstracts will be provided with Digital Object Identifier by