Theme: New research to uncover opportunities in the medical and pharmaceutical studies
Med Pharma Congress 2018
Conference Series llc LTD invites all the participants across the globe to attend 17th Annual Pharmaceutical Sciences and Medicinal Chemistry Congress during July 05-06, 2018 in Bangkok, Thailand, which includes prompt keynote presentations, Oral talks, Poster presentations and Exhibitions. Med Pharma Congress is a specially designed cluster Pharma conference. The main theme of this Pharma conferences is “New research to uncover opportunities in the medical and pharmaceutical studies” which covers a wide range of critically important sessions.
Track 1: Pharmaceutical sciences:
The pharmaceutical sciences are a group of interdisciplinary areas of study concerned with the design, action, delivery and disposition of drugs. They apply knowledge from chemistry (inorganic, physical, biochemical and analytical), biology (anatomy, physiology, biochemistry, cell biology and molecular), epidemiology, statistics, chemo metrics, mathematics, physics, and chemical engineering.
As new discoveries advance and extend the pharmaceutical sciences, subspecialties continue to be added to this list. Importantly, as knowledge advances, boundaries between these specialty areas of pharmaceutical sciences are beginning to blur. Many fundamental concepts are common to all pharmaceutical sciences. These shared fundamental concepts further the understanding of their applicability to all aspects of pharmaceutical research and drug therapy.
Related Pharma Societies | Pharma Associations
Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry, Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association (PIPA), Pharmaceutical Society Of New Zealand (PSNZ), Pharmaceutical Association of Mauritius(PAM), International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT)
Track 2: Pharmacology and Pharmacovigilance:
Pharmacology is the branch of medicine and biology concerned with the study of drug action, where a drug can be broadly defined as any man-made, natural, or endogenous (from within body) molecule which exerts a biochemical and/or physiological effect on the cell, tissue, organ, or organism (sometimes the word pharmacon is used as a term to encompass these endogenous and exogenous bioactive species). More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function. If substances have medicinal properties, they are considered pharmaceuticals.
Pharmacovigilance, also known as drug safety, is the pharmacologicalscience relating to the collection, detection, assessment, monitoring, and prevention of adverse effects with pharmaceutical products. The etymological roots for the word "pharmacovigilance" are: pharmakon (Greek for drug) and vigilare (Latin for to keep watch). As such, pharmacovigilance heavily focuses on adverse drug reactions, or ADRs, which are defined as any response to a drug which is noxious and unintended, including lack of efficacy (the condition that this definition only applies with the doses normally used for the prophylaxis, diagnosis or therapy of disease, or for the modification of physiological disorder function was excluded with the latest amendment of the applicable legislation). Medication errors such as overdose, and misuse and abuse of a drug as well as drug exposure during pregnancy and breast feeding, are also of interest, even without an adverse event, because they may result in an adverse drug reaction.
Related Pharma Societies | Pharma Associations
Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry, Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association (PIPA), Pharmaceutical Society Of New Zealand (PSNZ), Pharmaceutical Association of Mauritius(PAM), International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT)
Track 3: Pharmaceutical Engineering
Pharmaceutical engineering is a branch of pharmaceutical science and technology that involves development and manufacturing of products, processes, and components in the pharmaceuticals industry. While developing pharmaceutical products involves many interrelated disciplines like medicinal chemists, analytical chemists, clinicians/pharmacologists, pharmacists, chemical engineers, biomedical engineers, etc, the specific subfield of "pharmaceutical engineering" has only emerged recently as a distinct engineering discipline. This now brings the problem-solving principles and quantitative training of engineering to complement the other scientific fields already involved in drug development.
Pharmaceutical engineering is a branch of pharmaceutical science and technology that involves development and manufacturing of products, processes, and components in the pharmaceuticals industry.
Related Pharma Societies | Pharma Associations
Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry, Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association (PIPA), Pharmaceutical Society Of New Zealand (PSNZ), Pharmaceutical Association of Mauritius(PAM), International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT)
Track 4: Bio Pharmaceuticals:
A biopharmaceutical, also known as a biological medical product, biological, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semi synthesized from biological sources. Different from totally synthesized pharmaceuticals, they include vaccines, blood, blood components, allergenic, somatic cells, gene therapies, tissues, recombinant therapeutic protein, and living cells used in cell therapy. Biologics can be composed of sugars, proteins, or nucleic acids or complex combinations of these substances, or may be living cells or tissues. They (or their precursors or components) are isolated from living sources—human, animal, plant, fungal, or microbial.
Terminology surrounding biopharmaceuticals varies between groups and entities, with different terms referring to different subsets of therapeutics within the general biopharmaceutical category. Some regulatory agencies use the terms biological medicinal products or therapeutic biological product to refer specifically to engineered macromolecular products like protein- and nucleic acid-based drugs, distinguishing them from products like blood, blood components, or vaccines, which are usually extracted directly from a biological source. Specialty drugs, a recent classification of pharmaceuticals, are high-cost drugs that are often biologics. Gene-based and cellular biologics, for example, often are at the forefront of biomedical research, and may be used to treat a variety of medical conditions for which no other treatments are available.
Related Pharma Societies | Pharma Associations
Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry, Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association (PIPA), Pharmaceutical Society Of New Zealand (PSNZ), Pharmaceutical Association of Mauritius(PAM), International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT)
Track 5: Pharmacogenomics
Pharmacogenomics is the study of the role of the genome in drug response. Its name reflects it’s combining of pharmacology and genomics. Pharmacogenomics can be defined as the technology that analyzes how the genetic makeup of an individual affects his/her response to drugs. It deals with the influence of acquired and inherited genetic variation on drug response in patients by correlating gene expression or single-nucleotide polymorphisms with pharmacokinetics and pharmacodynamics (drug absorption, distribution, metabolism, and elimination), as well as drug receptor target effects. The term pharmacogenomics is often used interchangeably with pharmacogenetics. Although both terms relate to drug response based on genetic influences, pharmacogenetics focuses on single drug-gene interactions, while pharmacogenomics encompasses a more genome-wide association approach, incorporating genomics and epigenetics while dealing with the effects of multiple genes on drug response.
Pharmacogenomics aims to develop rational means to optimize drug therapy, with respect to the patients' genotype, to ensure maximum efficacy with minimal adverse effects. Through the utilization of pharmacogenomics, it is hoped that pharmaceutical drug treatments can deviate from what is dubbed as the "one-dose-fits-all" approach. It attempts to eliminate the trial-and-error method of prescribing, allowing physicians to take into consideration their patient's genes, the functionality of these genes, and how this may affect the efficacy of the patient's current or future treatments (and where applicable, provide an explanation for the failure of past treatments). Such approaches promise the advent of precision medicine and even personalized medicine, in which drugs and drug combinations are optimized for narrow subsets of patients or even for each individual's unique genetic makeup. Whether used to explain a patient's response or lack thereof to a treatment, or act as a predictive tool, it hopes to achieve better treatment outcomes, greater efficacy, minimization of the occurrence of drug toxicities and adverse drug reactions (ADRs). For patients who have lack of therapeutic response to a treatment, alternative therapies can be prescribed that would best suit their requirements. In order to provide pharmacogenomics recommendations for a given drug, two possible types of input can be used: genotyping or exome or whole genome sequencing. Sequencing provides many more data points, including detection of mutations that prematurely terminate the synthesized protein.
Related Pharma Societies | Pharma Associations
Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry, Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association (PIPA), Pharmaceutical Society Of New Zealand (PSNZ), Pharmaceutical Association of Mauritius(PAM), International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT)
Track 6: Pharmacognosy and Phytochemistry
Pharmacognosy is the study of medicinal drugs derived from plants or other natural sources. The American Society of Pharmacognosy defines Pharmacognosy as "the study of the physical, chemical, biochemical and biological properties of drugs, drug substances or potential drugs or drug substances of natural origin as well as the search for new drugs from natural sources. It is also defined as the study of crude drugs.
Phytochemistry is the study of phytochemicals, which are chemicals derived from plants. Specifically, Phytochemistry describes the large number of secondary metabolic compounds found in plants. Many of these are known to provide protection against insect attacks and plant diseases. They also exhibit a number of protective functions for human consumers. Phytochemistry can be considered sub-fields of botany or chemistry. Activities can be led in botanical gardens or in the wild with the aid of ethno botany. The applications of the discipline can be for Pharmacognosy, or the discovery of new drugs, or as an aid for plant physiology studies
Global sales of plant products was totally estimated us $60 billion in 2002 and is expected to get higher at 6.4% average growth rate.
Related Pharma Societies | Pharma Associations
Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry, Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association (PIPA), Pharmaceutical Society Of New Zealand (PSNZ), Pharmaceutical Association of Mauritius(PAM), International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT)
Track 7: Pharmaceutical Toxicology
Pharmaceutical Toxicology explains the methodology and requirements of pre-clinical safety assessments of new medicines. With the focus on medicinal drugs, the most important safety issues of drugs are covered. This includes registration requirements of new drugs and pharmacovigilance.
Pharmaceutical Toxicology is focusing on the diagnosis, management and prevention of poisoning and other adverse health effects due to medications, occupational and environmental toxicants, and biological agents. Medical toxicologists are involved in the assessment and treatment of acute or chronic poisoning, adverse drug reactions (ADR), overdoses, envenomation’s, and substance abuse, and other chemical exposures.
Related Pharma Societies | Pharma Associations
Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry, Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association (PIPA), Pharmaceutical Society Of New Zealand (PSNZ), Pharmaceutical Association of Mauritius(PAM), International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT)
Track 8: Industrial Pharmacy
Industrial Pharmacy also plays a crucial role in any drug discovery. To any novel drug discovery the industrial approach is very important to get massive commercial application. Few things which have to be considered by industries to provide a safe and cost affective medicine to the patients like Supply chain, Waste management, Product management, Post- marketing surveillance, Good manufacturing practices and Marketing.
The U.S. pharmaceutical market is the world’s most important national market. Together with Canada and Mexico, it represents the largest continental Pharma market worldwide. The United States alone holds some 40 percent of the global pharmaceutical market. In 2014, this share was valued around 365 million U.S. dollars. Many of the global top companies are located in the United States. In 2014, six out of the top eleven companies were U.S.-based.
Related Pharma Societies | Pharma Associations
Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry, Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association (PIPA), Pharmaceutical Society Of New Zealand (PSNZ), Pharmaceutical Association of Mauritius(PAM), International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT)
Track 9: Regulatory Affairs
Regulatory affairs (RA), is also called government affairs, is a profession within regulated industries, such as pharmaceuticals, medical devices, energy, banking, telecom etc. Regulatory affairs also has a very specific meaning within the healthcare industries (pharmaceuticals, medical devices, biologics and functional foods)
Regulatory affairs (medical affairs) professionals (aka regulatory professionals) usually have responsibility for the following general areas: Ensuring that their companies comply with all of the regulations and laws pertaining to their business, Working with federal, state and local regulatory agencies and personnel on specific issues affecting their business. i.e. working with such agencies as the Food and Drug Administration or European Medicines Agency (pharmaceuticals and medical devices); The Department of Energy; or the Securities and Exchange Commission (banking), Advising their companies on the regulatory aspects and climate that would affect proposed activities. i.e. describing the "regulatory climate" around issues such as the promotion of prescription drugs and Sarbanes-Oxley compliance.
Related Pharma Societies | Pharma Associations
Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry, Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association (PIPA), Pharmaceutical Society Of New Zealand (PSNZ), Pharmaceutical Association of Mauritius(PAM), International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT)
Track 10: Pharmaceutical Formulation
Pharmaceutical formulation, in pharmaceutics, is the process in which different chemical substances, including the active drug, are combined to produce a final medicinal product. The word formulation is often used in a way that includes dosage form. Formulation studies involve developing a preparation of the drug which is both stable and acceptable to the patient. For orally administered drugs, this usually involves incorporating the drug into a tablet or a capsule. It is important to make the distinction that a tablet contains a variety of other potentially inert substances apart from the drug itself, and studies have to be carried out to ensure that the encapsulated drug is compatible with these other substances in a way that does not cause harm, whether direct or indirect.
Preformulation involves the characterization of a drug's physical, chemical, and mechanical properties in order to choose what other ingredients (excipients) should be used in the preparation. In dealing with protein pre-formulation, the important aspect is to understand the solution behavior of a given protein under a variety of stress conditions such as freeze/thaw, temperature, shear stress among others to identify mechanisms of degradation and therefore its mitigation. Formulation studies then consider such factors as particle size, polymorphism, PH, and solubility, as all of these can influence bioavailability and hence the activity of a drug. The drug must be combined with inactive ingredients by a method which ensures that the quantity of drug present is consistent in each dosage unit e.g. each tablet. The dosage should have a uniform appearance, with an acceptable taste, tablet hardness, or capsule disintegration.
Related Pharma Societies | Pharma Associations
Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry, Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association (PIPA), Pharmaceutical Society Of New Zealand (PSNZ), Pharmaceutical Association of Mauritius(PAM), International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT)
Track 11: Nano Medicine
Nano medicine is the medical application of nanotechnology. Nano medicine ranges from the medical applications of #nanomaterial’s and biological devices, to Nano electronic biosensors, and even possible future applications of molecular #nanotechnology such as biological machines. Current problems for Nano medicine involve understanding the issues related to toxicity and environmental impact of Nano scale materials (materials whose structure is on the scale of nanometers, i.e. billionths of a meter). Functionalities can be added to nanomaterial by interfacing them with biological molecules or structures. The size of nanomaterial’ is similar to that of most biological molecules and structures; therefore, nanomaterial’s can be useful for both in vivo and in vitro biomedical research and applications. Thus far, the integration of nanomaterial with biology has led to the development of diagnostic devices, contrast agents, analytical tools, physical therapy applications, and drug delivery vehicles.
Nano medicine seeks to deliver a valuable set of research tools and clinically useful devices in the near future. The National Nanotechnology Initiative expects new commercial applications in the pharmaceutical industry that may include advanced drug delivery systems, new therapies, and in vivo imaging. Nano medicine research is receiving funding from the US National Institutes of Health, including the funding in 2005 of a five-year plan to set up four Nano medicine centers. Nano medicine sales reached $16 billion in 2015, with a minimum of $3.8 billion in nanotechnology R&D being invested every year. Global funding for emerging nanotechnology is increased by 45% per year in recent years, with product sales exceeding $1 trillion in 2013. As the Nano medicine industry continues to grow, it is expected to have a significant impact on the economy.
Related Pharma Societies | Pharma Associations
Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry, Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association (PIPA), Pharmaceutical Society Of New Zealand (PSNZ), Pharmaceutical Association of Mauritius(PAM), International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT)
Track 12: Analytical and Bio analytical Techniques
An #analytical technique is a method that is used to determine the concentration of a chemical compound or chemical element. There are a wide variety of techniques used for analysis, from simple weighing (gravimetric analysis) to titrations (titrimetric) to very advanced techniques using highly specialized instrumentation. Bio analysis is a sub-discipline of analytical chemistry covering the quantitative measurement of xenobiotic (drugs and their #metabolites, and biological molecules in unnatural locations or concentrations) and biotic (#macromolecules, proteins, DNA, large molecule drugs, metabolites) in biological systems.
Many scientific endeavors are dependent upon accurate quantification of drugs and endogenous substances in biological samples; the focus of bio analysis in the pharmaceutical industry is to provide a quantitative measure of the active drug and/or its metabolite(s) for the purpose of pharmacokinetics, toxicokinetics, bioequivalence and exposure–response (pharmacokinetics/pharmacodynamics studies). Bio analysis also applies to drugs used for illicit purposes, forensic investigations, anti-doping testing in sports, and environmental concerns. Bio analysis was traditionally thought of in terms of measuring small molecule drugs. However, the past twenty years has seen an increase in biopharmaceuticals (e.g. proteins and peptides), which have been developed to address many of the same diseases as small molecules. These larger biomolecules have presented their own unique challenges to quantification.
Related Pharma Societies | Pharma Associations
Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry, Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association (PIPA), Pharmaceutical Society Of New Zealand (PSNZ), Pharmaceutical Association of Mauritius(PAM), International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT)
Track 13: Drug designing and Drug Delivery
Drug design, often referred to as rational drug design or simply rational design, is the inventive process of finding new medications based on the knowledge of a biological target. The drug is most commonly an organic small molecule that activates or inhibits the function of a biomolecule such as a protein, which in turn results in a therapeutic benefit to the patient. In the most basic sense, drug design involves the design of molecules that are complementary in shape and charge to the bio moleculartarget with which they interact and therefore will bind to it. Drug design frequently but not necessarily relies on computer modeling techniques. This type of modeling is sometimes referred to as computer-aided drug design. Finally, drug design that relies on the knowledge of the three-dimensional structure of the bio molecular target is known as structure-based drug design. In addition to small molecules, biopharmaceuticals and especially therapeutic antibodies are an increasingly important class of drugs and computational methods for improving the affinity, selectivity, and stability of this protein-based therapeutics have also been developed.
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes pre-clinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug.
Related Pharma Societies | Pharma Associations
Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry, Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association (PIPA), Pharmaceutical Society Of New Zealand (PSNZ), Pharmaceutical Association of Mauritius(PAM), International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT)
Track 14: Clinical Trials and Clinical Pharmacy:
Clinical pharmacy is the branch of pharmacy in which pharmacists provide patient care that optimizes the use of medication and promotes health, wellness, and disease prevention. Clinical pharmacists care for patients in all health care settings but the clinical pharmacy movement initially began inside hospitals and clinics. Clinical pharmacists often work in collaboration with physicians, nurse practitioners, and other healthcare professionals.
Clinical trials are experiments done in clinical research. Such prospective biomedical or behavioral research studies on human participants are designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial - their approval does not mean that the therapy is 'safe' or effective, only that the trial may be conducted.
Related Pharma Societies | Pharma Associations
Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry, Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association (PIPA), Pharmaceutical Society Of New Zealand (PSNZ), Pharmaceutical Association of Mauritius(PAM), International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT)
Track 15: Digitization of Pharma:
The pharmaceuticals and life sciences industry has been very cautious in applying digital technology to improve manufacturing and supply chain operations thus far, yet that caution is becoming a hindrance. As the Pharma industry faces growing challenges including globalization, great supply chain complexity, price and cost pressure, and personalized medicine, among others digitization holds tremendous potential in helping companies adapt.
The pharmaceuticals and life sciences industry is generally quite advanced in its application of technology. Regarding operations, however, the industry has been very cautious in adopting new technologies thus far, still relying on supply chain and manufacturing paradigms that have been around for a long time. One key reason is the regulatory environment in which Pharma companies operate. High margins have traditionally been another reason, leading to a stronger focus on new product development and sales rather than on optimizing operations.
Related Pharma Societies | Pharma Associations
Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry, Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association (PIPA), Pharmaceutical Society Of New Zealand (PSNZ), Pharmaceutical Association of Mauritius(PAM), International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT)
Track 16: Intellectual Property Rights (IPR)
Intellectual property (IP) refers to creations of the intellect for which a monopoly is assigned to designated owners by law. Intellectual property rights (IPR) are the protections granted to the creators of IP, and include trademarks, copyright, patents, industrial design rights, and in some jurisdictions trade secrets. Artistic works including music and literature, as well as discoveries, inventions, words, phrases, symbols, and designs can all be protected as intellectual property.
While intellectual property law has evolved over centuries, it was not until the 19th century that the term intellectual property began to be used, and not until the late 20th century that it became commonplace in the majority of the world.
Related Pharma Societies | Pharma Associations
Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry, Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association (PIPA), Pharmaceutical Society Of New Zealand (PSNZ), Pharmaceutical Association of Mauritius(PAM), International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT)
Conference Series llc LTD invites all the participants across the globe to attend 17th Annual Medicinal and Pharmaceutical Sciences Congress during July 05-06, 2018 in Bangkok, Thailand which includes prompt keynote presentations, Oral talks, Poster presentations and Exhibitions. Med Pharma Congress is a specially designed cluster Pharmaceutical and Medicinal Chemistry Conference. The main theme of this Pharma conferences is “New research to uncover opportunities in the medical and pharmaceutical studies ” which covers a wide range of critically important sessions.
Why to Attend???
Meet Your Target Market with members from around the world focused on learning about Pharmaceutical Sciences and Medicinal Chemistry, this is your single best opportunity to reach the largest assemblage of participants from the Pharma and medicinal community, Find out how innovators are closing the gap between product development and adoption, Debate the potential to greater cross-industry and cross-sector collaboration in the race for faster, cheaper, better cures. Conduct demonstrations, distribute information, meet with current and potential Scientists, make a splash with a new research, and receive name recognition at this 2-day event Pharmaceutical Science & Medicinal Chemistry. World-renowned speakers, the most recent techniques, tactics, and the newest updates in Pharmaceutical Sciences fields are hallmarks of this conference.
A Unique Opportunity for Advertisers and Sponsors at this International event:
Target Audience:
- Professors, Associate Professors, Assistant Professors
- PhD Scholars
- Graduates and Post Graduates
- Directors, CEO’s of Organizations
- Association, Association presidents and professionals
- Noble laureates in Health Care and Medicine
- Bio instruments Professionals
- Bio-informatics Professionals
- Software development companies
- Research Institutes and members
- Supply Chain companies
- Manufacturing Companies
- CRO and DATA management Companies
- Training Institutes
- Business Entrepreneur
- Government healthcare departments, HTA’s and drug regulators
- Digital health companies
- Genomics and personalised medicine experts
The Pharmaceutical Industry's long successful strategy of placing big bets on a few molecules, promoting them heavily and turning them into blockbusters worked well for many years. But with span of time R&D productivity has now plummeted and the environment is changing. Seven major trends that are reshaping the marketplace:
- Instances of chronic disease are increasing, placing even greater pressure on already stretched healthcare budgets.
- Healthcare policy-makers and payers are increasingly mandating what doctors can prescribe.
- A growing number of healthcare payers are measuring the pharmacoeconomic performance of different medicines. A widespread use of electronic medical records will give them the data they need to insist on outcomes-based pricing.
- Boundaries between different forms of healthcare are blurring, as clinical advances render previously fatal diseases chronic and the self-medication sector expands.
- Demand for medicines is growing more rapidly in the emerging economies than the industrialized economies.
- Governments are beginning to focus on prevention rather than treatment, although they have not yet invested very much in pre-emptive measures; and
- Regulators are becoming more cautious about approving truly innovative medicines.
These trends will compound the challenges faced by Pharma sector, but they’ll also provide some major opportunities.
Global Pharma Market:
The overall $1.12 trillion Pharma market in 2022, will rise at a faster clip during 2016-2020, and then slow down a bit as major patent expirations take hold. Evaluation says that the global market actually declined by 1.0% in 2015, and by 4.8% in 2017. Prescription sales excluding generics has risen 4.4% in 2017, and will reach $1.006 trillion in 2022. Generics sales will increase from $73 billion in 2015 to $115 billion in 2022, and constitute 10.2% of prescription sales at that point (only 0.3 percentage points more than it is now).
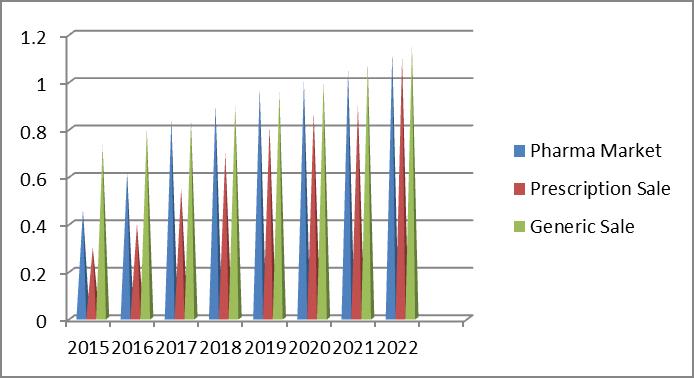
During the 2008-2015 period, the compound annual growth rate of global R&D spending was 1.7%; during the 2016-2022 periods, the rate will grow at 2.8%. The year-over-year increase, however, will remain around 3%, versus some dramatic jumps seen in 2013, 2014 and 2015. Overall spending will reach $182 billion in 2022.
Asia Pharma Market:
Asia-Pacific has shown a tremendous growth in past years which is driven by several factors, the most important of which is the technological innovations. Technological innovations and advancements have led to the introduction of new products. The other factors promoting the growth of the market are increasing incidences of chronic diseases, and diabetes patients, ease of use and interpretation of medical devices. Factors constraining the growth of the market include reimbursement issues, high cost of the medical devices.
The Asia-Pacific medical wearable electronics market was worth more than $1.2billion in revenue in 2016 and is expected to cross $3.01 billion in 2021, growing at a healthy CAGR of 26.19% from 2016 to 2021.
Thailand Pharma Market:
According to the International Monetary Fund (IMF), Thailand’s GDP based on Purchasing Power Parity (PPP) exceeded $1.1 trillion dollars in 2016, making Thailand the second largest economy in Southeast Asia after Indonesia, which has a GDP (PPP) of $3 trillion. In 2016, Thailand’s GDP grew by 3.2%, compared to 1.6% in the US and 2% in the European Union (IMF). The Asian Development Bank predicts Thailand’s GDP will grow 3.5% and 3.6% in 2017 and 2018, respectively.
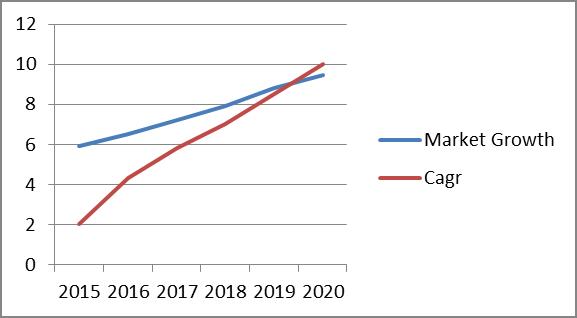
Thailand imported over $2.2 billion in pharmaceuticals in 2016, a considerable increase from $1.8 billion just two years ago. In 2016, over 65% of Thailand’s pharmaceutical imports came from the United States, Europe, and Canada. Germany was the largest pharmaceutical exporter to Thailand, followed by the US, France, then Switzerland. The Thai pharmaceutical market was valued at over $5 billion in 2016, making it the second largest market in Southeast Asia. The value of Thailand’s pharmaceutical market is expected to double by 2020. Pharmaceutical sales per-capita is also predicted to grow from $75 in 2016 to $125 by 2024.
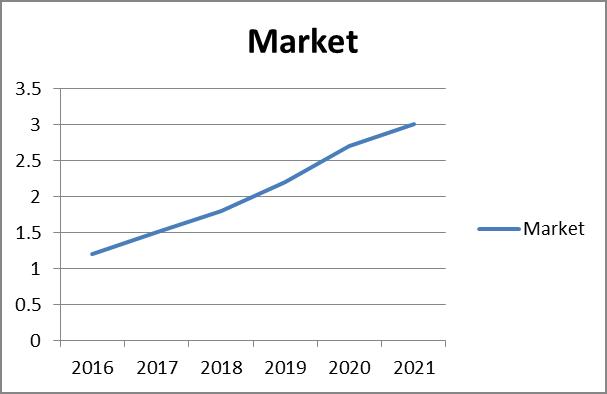
The pharmaceutical market in Thailand is expected to rise from $5.91 billion in 2015 to $9.47 billion by 2020, increasing at a projected CAGR of 10%.
Conference Highlights
- Pharmaceutical sciences
- Pharmacology and Pharmacovigilance
- Pharmaceutical Engineering
- Bio Pharmaceuticals
- Pharmacogenomics
- Pharmacognosy and Phytochemistry
- Pharmaceutical Toxicology
- Industrial Pharmacy
- Regulatory Affairs
- Pharmaceutical Formulation
- Nano Medicine
- Analytical and Bio Analytical Techniques
- Drug designing and Drug Delivery
- Clinical Pharmacy and Clinical Trials
- Digitization of Pharma
- Intellectual Property Rights (IPR)
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | July 05-06, 2018 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | ||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
Abstracts will be provided with Digital Object Identifier by



















